Proving Secure DNA Sample Containment of a High-Efficiency PCR Molecular Diagnostic System
 Just like many other industries today, the medical field is adapting more automated technology and systems to improve efficiency and eliminate guesswork. Some diagnostic systems, such as ones used for polymerase chain reaction (PCR) processes, have been around for many years and are available in a range in configurations and unit sizes.
Just like many other industries today, the medical field is adapting more automated technology and systems to improve efficiency and eliminate guesswork. Some diagnostic systems, such as ones used for polymerase chain reaction (PCR) processes, have been around for many years and are available in a range in configurations and unit sizes.
Speed is an important value proposition to many of these types of systems – especially when it comes to reducing operator hands-on time.
In a PCR process, DNA molecules are inserted into individual compartments within a pressurized cartridge, and heated to a high temperature to melt/separate the DNA strands. Within each compartment, a solution including DNA polymerase – which is heat resistant – works to synthesize the separated DNA strands and create many millions of synthesized strands from just a single sample. As this article from the Khan Academy explains, DNA amplified by PCR may be sent for sequencing, visualized by gel electrophoresis, or cloned into plasmid for other experiments.
Designing a PCR molecular diagnostic system with minimal hands-on time requires assurance that all hands-free parts of the design will perform to exact expectations, with regards to the safe and efficient handling of patient DNA samples. The more embedded automated features designed into these systems, the greater the amount of testing is required during the prototyping phase.
As one molecular diagnostic system manufacturer found out, Tekscan pressure mapping technology can be an insightful R&D tool to quantify how automated compartments interact within the unit during the while evaluating a new machine design.
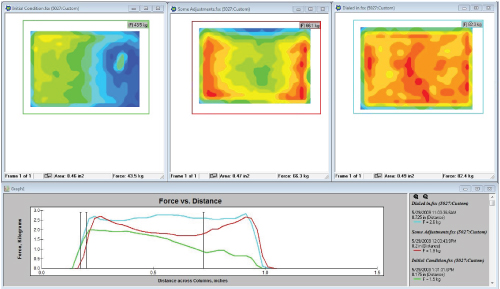 The left-most image shows initial pressure distribution of the cartridge while pressed against the sealing plate. The middle image shows results after adjustments were made, while the right-most image shows ideal pressure distribution across the cartridge.After DNA samples are placed within the system, a cartridge containing individual compartments of DNA samples is closed in a drawer, and pressed against a plate that applies heat. In this particular application, the samples were pressed against the plate for over an hour. Even interface pressure and 100% contact is a corollary to even heat transfer from the machine to the cartridge. They used an I-Scan system to ensure complete contact, and that the interface pressure across the cartridge was as even as possible.
The left-most image shows initial pressure distribution of the cartridge while pressed against the sealing plate. The middle image shows results after adjustments were made, while the right-most image shows ideal pressure distribution across the cartridge.After DNA samples are placed within the system, a cartridge containing individual compartments of DNA samples is closed in a drawer, and pressed against a plate that applies heat. In this particular application, the samples were pressed against the plate for over an hour. Even interface pressure and 100% contact is a corollary to even heat transfer from the machine to the cartridge. They used an I-Scan system to ensure complete contact, and that the interface pressure across the cartridge was as even as possible.
After first experimenting with a standard pressure mapping sensor, a custom pressure mapping sensor was designed to the exact dimensions of the DNA cartridge. The custom design ensured that the sensor would not move while the drawer containing the DNA cartridge closed. With the sensor in place, the design engineers could monitor pressure distribution across the entire cartridge for the duration of the test, making adjustments to the instrumentation to accommodate areas of peak pressure.
Similar applications could also be used as a field-service or machine-setup tool, or even as an embedded application within the system to prove even contact before processing begins.
Have a unique application that calls for a unique sensor? Visit this page to learn a little more about our sensor customization process.