Top 5 Reasons Why Medical Device Manufacturers Trust FlexiForce
 As a medical device developer, you’re often asked to balance more design challenges than other engineers may face. Not only do your device designs have to account for key usability and safety considerations, but the long, laborious, and often expensive regulatory process requires being efficient while maintaining a high level of detail.
As a medical device developer, you’re often asked to balance more design challenges than other engineers may face. Not only do your device designs have to account for key usability and safety considerations, but the long, laborious, and often expensive regulatory process requires being efficient while maintaining a high level of detail.
Trial and error may be an inherent part of the R&D process, but company investors may not be as forgiving when design flaws or poor choices of embedded technology hold up a device’s path to approval. Collaborating with a technology provider that has a successful track record – especially in the area of sensors for medical device design – is essential for avoiding these costly mistakes.
Therefore, here are the Top 5 Reasons medical device manufacturers trust FlexiForce™ touch sensors as an embedded force-sensing technology.
Reason 1 - Quality
All manufacturing takes place at ISO 9001: 2008 compliant & 13485: 2016 registered Tekscan headquarters. All of our sensors are 100% tested for quality, giving customers assurance that each sensor will perform as intended.
Reason 2 - Superior Sensor Properties
FlexiForce touch sensors have proven to have better force sensing properties than similar force-sensitive resistors. This includes linearity, repeatability, hysteresis, and drift.
Reason 3 - Power Efficiency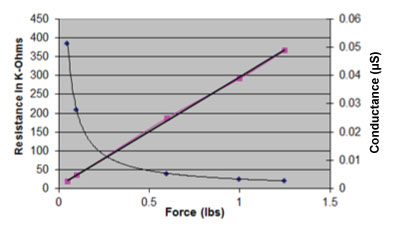
The FlexiForce sensor is a passive component. As shown in this graph, when the sensor is unloaded, the resistance is high (MΩ) and drops to the low KΩ when fully loaded. This large dynamic range allows for the design engineer to use simple electronics that do not require a lot of filtering. The conductance (1/R) is linear. These factors combine to result in low power requirements, making the FlexiForce sensor ideal for embedding into small, portable medical devices that don’t utilize much power.
Reason 4 - Customizable Solutions
Not only are FlexiForce touch sensors thin, flexible, and non-invasive, but the technology is quite customizable to most any product design, and is well-suited to common medical device operating conditions. Click here for a deeper look into the sensor customization process.
Some of our manufacturing capabilities include:
- Sensing area size: FlexiForce sensors have been manufactured with active area diameters as small as 1.86 mm (0.04 in) and widths as large as 402.56 mm (15.85 in)
- Pressure range: Some sensor options reaching up to 10,000 psi (1,700 bar)
- Operating temperature: Sensor options available to withstand up to 200°C (400°F)
- Sensing area uniformity: When compared to other ultra-thin force sensor (or, Shunt Mode sensor technology), FlexiForce sensors will provide consistent output regardless of where on the sensor the load is applied. This article provides more comparison points between FlexiForce and Shunt Mode sensors.
Reason 5 - Experience & Stability
From large global medical device manufacturers, to small start-ups, the partnerships Tekscan has forged with medical device OEMs is a major reason why the company has remained stable for decades. Our team of Applications Engineers is committed to helping design engineers innovate their devices with force-feedback technology, while adhering to the design’s space, power, and regulatory challenges.
We're Standing By to Help You Succeed